Umnqalo wevanga
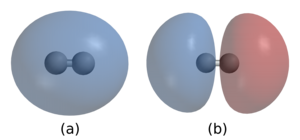
Umnqalo wevanga ungukuqalangana kwamaChwe noma izihonyo ukuze kwakheke imizwayi, imincwebe, nezinye izinhlaka. Umnqalo ungase ube yisisusa sesiphoqi sobuzuba ngaphakathi kwezihonyo ezizutshiwe njengasemnqalweni wesihonyo noma ngokwabelana ngezinzuba njengasemnqalweni wezinzuba, noma ngenhlanganisela ethile yale miphumela. Iminqalo yevanga ichaziswa njengenamandla ahlukene: kukhona "iminqalo eqinile" noma "iminqalo emqoka" efana nomnqalo wezinzuba, wezihonyo kanye nowezinkimbi, kanye "neminqalo ebuthaka" noma "iminqalo eyisibili" efana nokunanana kwama-dipole, isiphoqi senhlakazo sase-London., kanye nomnqalo weHwanzi .
Njengoba imilelesa yamazuba ephikisanayo ihehena, izinzuba ezinomlelesa ophikayo ezizungeze inuzi kanye neziNelesi ezinomlelesa ovumayo ezingaphakathi kweNuzi ziyahehana. Izinzuba ezabiwayo phakathi kwezinhlayiyana zechwe ezimbili zizohehana enye kwenye. "Isiphazamiso sensebenzo yamaza ekuguxuzeleni kohoyana esakhayo" [1] siyazizinzisa izinhlayiyana zechwe eziphahliwe. Izinhlayiyana zechwe eziqalangene zizigcina ziseibangeni eliluganya (ibanga lomnqalo) kanjalo zilinganisa imithelela ehehayo nefuqayo echazwa ngokwenani emchachisweni wohoyana . [2] [3]
AmaChwe asemizwayini, emncwebeni, nasezinkimbini nezinye izinhlobo zoTho abanjwa ndawonye yiminqalo yevanga, anquma uhlaka kanye nezigubo zoTho.
- ↑ Levine, Daniel S.; Head-Gordon, Martin (2020-09-29). "Clarifying the quantum mechanical origin of the covalent chemical bond". Nature Communications (Springer Science and Business Media LLC) 11 (1): 4893. doi:10.1038/s41467-020-18670-8. ISSN 2041-1723. PMC 7524788. PMID 32994392. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=7524788.
- ↑ Pauling, L. (1931), "The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules", Journal of the American Chemical Society 53 (4): 1367–1400, doi:10.1021/ja01355a027
- ↑ Hund, F.. "Zur Deutung der Molekelspektren. IV". Zeitschrift für Physik 51 (11–12): 759–795. doi:10.1007/BF01400239.
